Summary
- Injectable Combination Products. Issues and challenges for industry.
- Deadline Extension or Not: Key Points for Class I Manufacturers
- The new challenges of injectable medicines
- Clinical Investigation of Combined Medical Devices Under New Regulation (EU) 2017/745 (MDR)
- Contribution of the physicochemical characterization of the materials constituting medical devices for rationalisation of their biological assessment.
- Steering Cleaning Validation Performance : A Key Industrial Challenge.
- Cleaning Process Validation: Why and How to Validate Analytical Methods and Related Sampling Methods
- A Risk-Based Approach to Stainless Steel Equipment Maintenance in cGMP Manufacturing Environment
- Single Use Systems vs Re-Usable Stainless-Steel Equipment. Compliance & Quality Perspective.
Single Use Systems vs Re-Usable Stainless-Steel Equipment. Compliance & Quality Perspective.
One of the current focus of Industry is how to define the “plant of the future”. During some conferences, among the all proposed tracks to improve production processes in term of:
• Flexibility
• Operating time and first to the market
The usage of SUS in aseptic processes, is often seen as the panacea.

This is based on the following assumptions (not an exhaustive list, some suppliers are quite more optimistic):
• No cleaning
• No sterilization step during operation
• Less maintenance and repair
• Absence of cross-contamination risk
• Faster to implement in processEven if these assumptions are most of the time true, we should not forget some important rules when jumping to an apparent simple solution. When eliminating some risks, some are added. Is SUS the process equipment panacea for the plant of the future?
1. Authorities expectations
1.1 Cleaning Requirements for Stainless Steel Equipment
What is the situation?
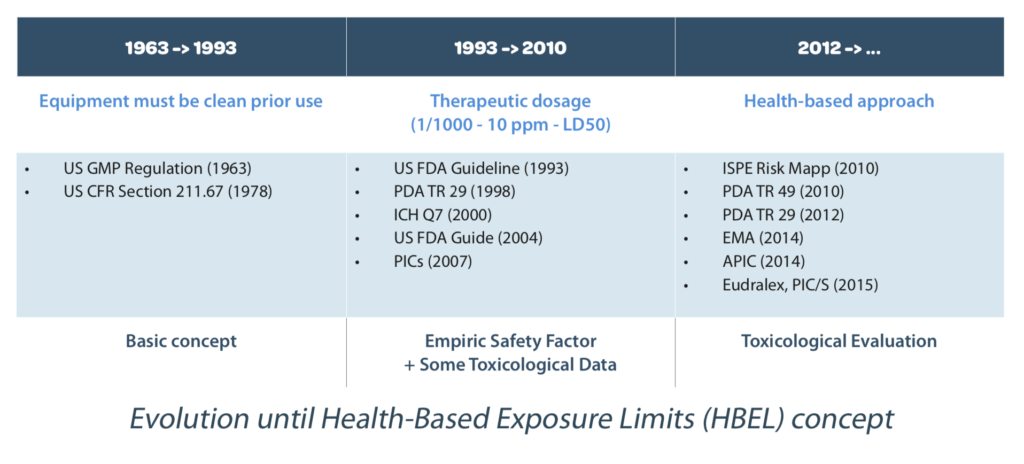
The evolution of the cleaning start with basic consideration to have clean and maintained equipment (generally visual inspection was enough) to high level of requirements taking into consideration:
• Cross-contamination and carryover
• Potential Cumulative effect of multiple items of equipment in the process equipment train (MACO calculation)
• Toxicological approach (Health-based approach with PDE calculation)
• Cleaning limits should be established for the removal of any cleaning agents used (and the removal of maintenance agents should also be evaluated).
• Different sampling techniques (Rinse, swab…)
• Adequate & validated analytical methods
• Visual inspection with qualification of operators
• Responsibility of contract givers: “Contract givers should either provide a full HBEL assessment to contract manufacturers or provide the data to allow the contract manufacturer to conduct the HBEL assessment “(Q&A EMA: Q5).
As mentioned by Patricia L. Alcock, FDA CDER and confirmed by new Eudralex: “relying only on visual examination would not be scientifically sound.”
1.2 Single Use Systems Requirements
Except general rules to ensure that the material of the SUS to be used in product manufacturing will not adulterate the product. In other words, the manufacture, processing, packing or holding with a SUS, are operated in accordance with Good Manufacturing Practice to assure that product is safe, and has the identity and strength, and meets the quality and purity characteristics which it purports or is represented to possess.
In fact, there is few regulatory requirements that mention directly SUS and current applicable expectations are based on Extractable &
Leachable Primary packaging requirements.
The absence of clear requirements in international reference could lead to a weak validation approach which is not in line with cGMP principle (When implementing a change, the quality of the product should not be downgraded). Then, the change from stainless steel design to SUS approach should ensure the same or higher level of quality (refer to previous chapter regarding cleaning requirements).
Consequently, the lack of specific regulatory requirements or expectations should be compensated by a higher level of scrutiny on SUS validation by quality department.
2. Single Use System qualification
As any manufacturing equipment, SUS must be fit for the intended use; to demonstrate it, a qualification exercise must be performed.
But at least, two main risks appear to be considered particularly for SUS:
2.1 SUS Manufacturing Process Control
To maintain the right level of control on the SUS manufacturing process, the following should be taken into considerations:
Assessment of the supplier with quality systems in place to ensure:
• Rapid investigation and appropriate communication of any deviations during manufacturing steps
• Adequate change management, to inform the customer of any potential impact on the SUS usage
• Strong process validation with a continued process verification in place, giving a monitoring of CQA and CPP that ensure that SUS batches are within the defined criteria and can be deemed identical.
• Well defined process to manage raw materials suppliers (the entire supply chain should be under control)
As the quality of the process equipment is delegated to a third party (SUS manufacturer), it is crucial to manage the quality through several controls (non-exhaustive):
• Regular destructive or non-destructive tests at manufacturing step (e.g. integrity test, mechanical resistance tests, tests for materials)
• Regular checks on received SUS batches (e.g. dimensional measures, intra-batch parameter)
• Audits of the SUS manufacturer.
2.2 Extractable & Leachable Considerations
In comparison with “old fashion stainless steel” approach, the main risk is not cross contamination but contamination from the SUS itself.
To evaluates the risk of contamination of product by the SUS, extractable assessment should consider specific elements like:
• Element that may increase the risk of having leachable substances (in kind and quantity) ;
• Process step where the SUS is used (filling vs early process steps) ;
• Contact time duration ;
• Presence of pre-treatment (e.g. sterilization, irradiation).
Other elements may reduce the final risk of having leachable substances (in kind and quantity):
• Potential dilution effect ;
• Presence of purification steps that may potentially eliminate some leached substances (need a demonstration by data generation).
The reference generally used for E&L studies and assessment is the USP 661 currently under revision to integrate and to precise the necessary aspect of SUS (future USP 665). However, as the implementation of this USP is not foreseen before 2025, meantime, companies have to anticipate and to define their own strategy based on current USP and the draft of USP 665/1665. They can also take into consideration documents edited by the BPOG (BioPhorum Operations Group).
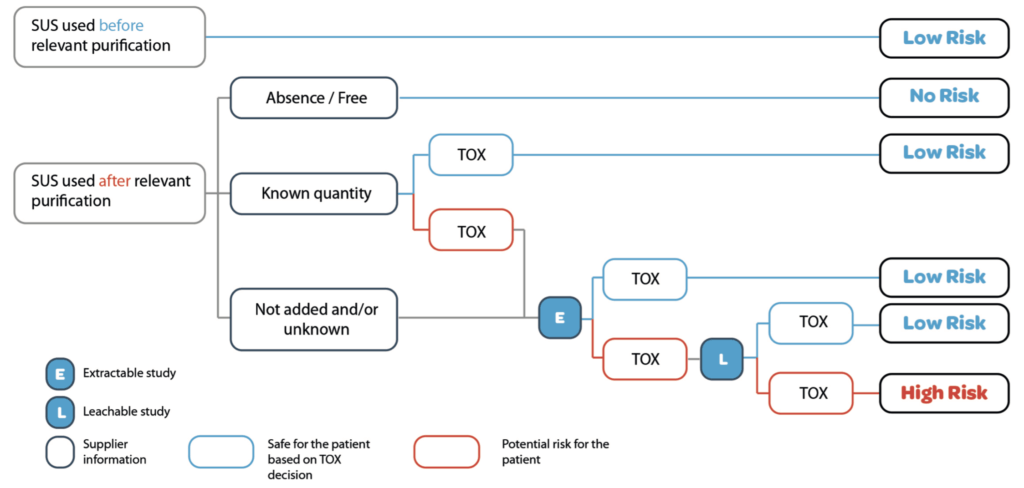
If a risk remains after extractable studies, leachable study should be performed to identify and to quantify the level of substance in the product. Toxicologist should determine the acceptable level.
The Figure 1, gives an example on how these elements should be assessed, the first step is to consider the risk to have the presence of Leachable, for this element the presence of relevant purification steps for the removal of potential Leachable should be used. However other elements should also be considered like contact time duration, type of product in contact with the SUS or the Material Reactivty. The second step is to collect data from supplier (e.g. additives) and assess these data. Based on these information, a toxicological evaluation could be launched on extractable and when needed on leachable to ensure that safety risks are well managed and under control.
3. Discussion
Practically, the increase of cleaning requirements in the past, were important but manageable as it was mainly re-development of cleaning cycles and some collateral impacts on equipment.
For SUS, the clarification or the increase of requirements (e.g. systematic extractable & leachable studies, cumulative effect assessment, toxicological evaluations, SUS manufacture under control – cPK, pPK) could bring a lot of burdens, if these new requirements are not planned in advance.
Building a drug manufacturing plant around SUS could reduce a lot the facility footprint. But as a consequence, in case of increase or clarification of authorities’ expectations, the re-design of the facility to come back to stainless steel equipment could hugely impact the drug manufacture in delay and budget (e.g. re-designed studies, building changes, walls re-construction, and requalification activities).
These potential new requirements may also lead to a shortage in supply as the implementation of the corrective actions could take time.
On top of the previous considerations, there is a consensus to start reducing the usage of single-use plastics products for which plastic- free alternatives already exist. Other measures aim to reduce the consumption of the most frequently littered plastic products; to extend the producers’ responsibility; to change the design of some products; and to inform and raise awareness among consumers.
Conclusion
The impact of an increase of authorities’ SUS requirements or clarification of them in a near future, could be huge on a plant designed with a large usage of SUS.
As an advice, before jumping into SUS “plant of the future”, consider the constraints related to SUS usage in the similar approach as the cleaning residues management.
Share article

Thierry Deflandre, Alexandre De Neef & Etienne Michel – GSK
thierry.d.deflandre@gsk.com ; alexandre.de-neef@gsk.com ; etienne.v.michel@gsk.com
Glossary
References
1978 (US CFR): Section 211.67 added describing equipment cleaning and maintenance
1993 (US FDA guideline): Guide to inspections for cleaning validation2000 (ICH Q7): Guide for active pharmaceutical ingredients
2004 (US FDA guide): Guide to inspections validation of cleaning processes
2010 (PDA): TR 49 – Points to consider for Biotechnology Cleaning Validation
2010 (ISPE): Risk-Based Manufacture of Pharmaceutical Products
2012 (PDA): TR 29 – Points to consider for Cleaning Validation
2015 (EMA): Guideline on setting health based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities
2015 (Eudralex Vol. 4 – GMP Guidelines Annex 15): Qualification and Validation